The Oxidative Capacity of the Atmosphere is Constant
The hydroxyl radical (HO, Eli's friend the air quality modeler insists it is HO, not OH, and Eli is a reasonable Rabett) is the vacuum cleaner of the atmosphere. Just about all the dirt (read volatile organics) gets cleaned up by OH HO. More precisely put, reaction with HO is the first step in the complete oxidation of organics to water and carbon dioxide. Eli discussed the basics in a post on atmospheric degradation of methane.
Starting in the 1970s a cottage industry grew up on direct measurement of HO concentrations by laser induced fluorescence from the ground, from airplanes, from ships but these measurements are limited by having a laser here, there and of course they are not everywhere at everytime. Other methods depend on measuring a stable (on the scale of a few years) tracer and following its concentrations. Because the concentration of HO is so low (it's very reactive, so it does not last long), it is not possible to measure from satellites, but it is important to know what the global concentration is because that determines the "oxidative capacity" of the atmosphere, which is basically how fast stuff like methane (a more potent greenhouse gas) gets transformed into CO2 (a sufficiently potent greenhouse gas), and if you don't care about that, it is also key to the photochemical generation and destruction of smog.
A recent paper in Science by Montzka, Krol, Dlugokencky, Hall, Joekel and Lelieveld (free viewing) describes an extension of the tracer method. The figure below from a commentary on that paper by Isaksen and Dalsøren shows how HO is generated and does its work.
Montzka, et al, looked at the loss of methyl chloroform (CH3CCl3) since 1998 which is a first order process, ie. directly proportional to [HO] (the square brackets mean concentration of)
where E is the global emission rate and G the total amount of the methyl choloroform. To do this properly requires that one know both the emission rates and the global burden, and knowing the emission rates is hard, but the Montreal Protocols ramped down production and there are no significant natural sources, so after 1998, emissions go to zero, and the decay becomes a simple exponential, the slope of which is directly proportional to global [HO]. If [HO] is constant, the rate constant will be constant
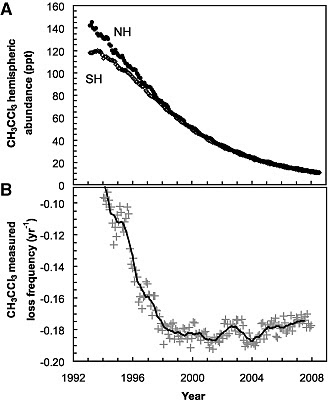 and the news is, why yes, it is constant after 1998, from which Montzka, et al. conclude that the interannual variability is small, about 2.3 ± 1.5% between 1998–2007. They believe that previous measurements were in error because of uncertainties in the amounts that were put into the atmosphere. The lag is probably related to the mixing time in the atmosphere, the time needed to mix the methyl cholorform completely and wipe out any gradients. [UPDATE: Clearly shown by the difference between the northern and southern hemisphere values up to ~1998.] There are the usual caveats, but taking Willis Eschenbach's advice, Eli simply says that the oxidative capacity of the atmosphere is constant.
and the news is, why yes, it is constant after 1998, from which Montzka, et al. conclude that the interannual variability is small, about 2.3 ± 1.5% between 1998–2007. They believe that previous measurements were in error because of uncertainties in the amounts that were put into the atmosphere. The lag is probably related to the mixing time in the atmosphere, the time needed to mix the methyl cholorform completely and wipe out any gradients. [UPDATE: Clearly shown by the difference between the northern and southern hemisphere values up to ~1998.] There are the usual caveats, but taking Willis Eschenbach's advice, Eli simply says that the oxidative capacity of the atmosphere is constant.One of the caveats, of course, is that it can get swamped but large emissions of things like methane. More on that later.





3 comments:
Dear prof. Eli, are blogs supposed to be used like this? This sounds like reporting science accurately, more like something I would expect to hear in primer to advanced climate studies in university. Well, I guess it's your blog and you do what you will in here, so I'm not bothering you with speculations, especially since I don't have numbers to back those up.
Yours, jyyh.
Perhaps brer rabbit can get Santa to help clean up. He has many HO's
As bunnies of a certain age will recall, Santa brought the miniature trains to generally older children (if younger, *never* give them two engines -- ask me how I know this, but I will not tell), and just a few years after that the yet older children could be heard chanting HO! HO! HO! throughout the campuses of the land. It must have warmed Santa's heart to hear.
Post a Comment