In Part I, the Spectroscopic Basis, Eli looked at measurements of the O2, N2 and CO2 spectra and found that the CO2 is absorption is many times stronger than the O2, and N2 absorption even taking into acount the much higher density of the diatomics . Strong enough that one can neglect the absorption of the other two molecules as a practical matter, however let the Bunny not stop there but go on to the quantum basis of all this trying not to get either too mathematical or too esoteric (esoteric comes in Part III: Putting the Pressure On where the surprises are). Eli will attempt to be correct, but not perfect and certainly not complete, that is a two semester course.
Starting back with neolithic quantum physics, let us now look at the emission spectrum of a blackbody. We can treat the surface of the Earth as one in the IR with unit emissivity (OK ice is a bit different but it is a lot colder so it emits a lot less) and look at the spectrum.we would expect at 290 K
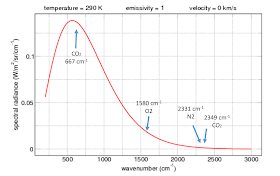
 where your gracious host has marked where our three players, O2, N2 and CO2 would absorb and what the black body emission would be at 290K. The observant among Eli's readers have noticed that there is simply no IR, or darn near none of it out where N2 and the CO2 asymmetric stretch absorbs.
where your gracious host has marked where our three players, O2, N2 and CO2 would absorb and what the black body emission would be at 290K. The observant among Eli's readers have noticed that there is simply no IR, or darn near none of it out where N2 and the CO2 asymmetric stretch absorbs.If you want to know what the bending and symmetric stretching vibrations are, make sure your significant other or keeper is not around. Place your fists on either side of your head and move them up and down or forward and back. Your head is the model of the C atom and the fists are oxygen. That is the bending vibration. There are two such, forward and back and up and down. They have the same frequency and Eli calls them degenerate. If somebunny catches you doing this, you may be so called also. For the asymmetric stretch move one of your fists toward your head and the other away while bending the noggin toward the fist that is trying to hit it. Then reverse. Folks doing this too enthusiastically can knock themselves out.
Rabett Run now needs to crawl a bit further down the physics tree to Electricity and Magnetism. Light (Eli will use the word light to describe IR, which strictly speaking annoys the fussbudgets who reserve light for visible light, but what the heck), is electromagnetic radiation, from the gamma ray to the radio waves and beyond in both directions.
Molecules are composed of atoms, which are composed of positively charged nuclei and negatively charged electrons. The charges have electric fields, which interact with each other and the electromagnetic field. The forces created by the interaction can move the charges relative to each other and in space.
We can describe the field created by the electrons and nuclei one by one or we can describe the potential energy in the field at a point a large distance r from the molecule as a power series in (1/r)n. If r is large, the importance of each term decreases with n. It is pretty well hopeless to describe the field created by the charges one by one especially if they are moving, and the power series converges quickly. That means that each term is a lot bigger than the next so in practice we only need to keep the first non zero term, maybe the second. This power series is called a multipole expansion.
 You can look up the details of the multipole expansion, but it is just a bunch of geometry where each charge qi is some distance ri from a point in space P which is quite far away. For convenience in what follows we can let the origin of the coordinate system be at the center of charge of the molecule.
You can look up the details of the multipole expansion, but it is just a bunch of geometry where each charge qi is some distance ri from a point in space P which is quite far away. For convenience in what follows we can let the origin of the coordinate system be at the center of charge of the molecule.The first term in the multipole expansion is proportional to the sum over all the charges divided by (r). Since molecules are neutral the sum of charges is zero and the first term is zero for a molecule.
The second term, for which the potential would vary as 1/r2 is called the electric dipole and is equal to Σ qi di where Σ is the sum over all the charges and di is a vector pointing towards charge i. Rather than bothering about the math, let's look at some examples CO2 and H2O.
For the electrons, the image is an electron anomaly distribution, with the blue areas being electron rich and the red electron poor, but the point is that the distribution is also cylindrically symmetric and for every point that contributes positively to to the electric dipole moment there is one that contributes negatively. They cancel, and the net electric dipole moment of ground state CO2 is zero.
The same is true for O2 and N2
The case for H2O is different. The shape of the molecule is bent, there is a region of high electron density on the side of the molecule facing away from the hydrogen atoms and the electron density on the side of the hydrogen atoms facing away from the oxygen atom is electron poor. H2O will have a permanent dipole moment. The blue arrow points towards the region of higher negative charge
When a molecule with a permanent dipole moment rotates (there are three axes that water vapor can rotate about) the dipole moment moves and that movement interacts with light because light is an electro-magnetic field that is influenced by moving dipoles and/or can influence them. By this mechanism rotating water vapor molecules can either gain (absorption) or lose (emission) light (IR or better put Far IR) between 0 and 800 cm-1 accompanied by a change in rotational state. Molecules with zero dipole moment can spin merrily on their way but they do not interact with light in this way. We can see these rotational transition in the spectrum of water vapor between 0 and 800 cm-1
The semi-log plot comes from another handy dandy web app Spectral Plot. The rotational lines (the bunch on the left) overlap the CO2 bending vibration as can be seen in the high resolution FIRST balloon specta taken at about 60 km looking down.
 The bunch of water vapor lines at 1700 cm-1 are the result of the bending vibrational transition. Looking at the electron density map of HOH, it is clear that changing the molecule bends the H-O-H angle will change, vibrating about the equilibrium ground state position as shown in this gif from Marc Henry.
The bunch of water vapor lines at 1700 cm-1 are the result of the bending vibrational transition. Looking at the electron density map of HOH, it is clear that changing the molecule bends the H-O-H angle will change, vibrating about the equilibrium ground state position as shown in this gif from Marc Henry. The instantaneous change in the dipole moment is called the transition dipole moment. It is the non-zero transition dipole moment that makes the bend (Source at UVA)
The instantaneous change in the dipole moment is called the transition dipole moment. It is the non-zero transition dipole moment that makes the bend (Source at UVA)
and asymmetric stretch of CO2 IR active,
while the symmetric stretch is not. A little thought will show that when homonuclear diatomic molecules such as N2 and O2 begin to vibrate there is no change in the dipole moment and therefore they cannot absorb or emit IR accompanied by a change in vibrational state.

A really good analogy to this is a dipole antenna such as the ones used for receiving FM radio.
So how do N2 and O2 interact with light? Well, the next element in the multipole expansion beyond the electric dipole moment is the quadrupole moment. Eli will not bother you with how to calculate it. It turns out that, both N2 and O2 have ferocious quadrupole transition moments, essentially because they are so cylindrically symmetric and the same is true of the symmetric stretch of CO2, but even with a strong quadrupole transition moment the fact that the interaction of a quadrupole with light is proportional to 1/r3 rather than 1/r2 makes their absorption much weaker. There is also an antenna analogy. The Adcock antenna, used for direction finding, is a quadrupole array.
The TL:DR version of this is that it is the interaction of the electromagnetic field of light with the charge distribution of molecules that gives rise to absorption and emission of IR radiation. Although not dealt with here, quantum mechanics tells us about what changes in rotational and vibrational levels are allowed if the electromagnetic interaction is non-zero. If the electric dipole is nonzero, vibrational transitions with unit change in quantum number are strongly favored, the same is true for rotational transitions. Overtones with changes of two or more quanta are very weak.
So stay tuned for the exciting finale.




"found that the CO2 absorption is like." Is like what?
ReplyDelete"many times stronger than the O2, and N2 absorption even taking into acount the much higher density of the diatomics"
ReplyDeleteProof reading is not always Eli's thing. Thanks.
This and other excellent explanations are being sent off to the good Judge Alsop or to the CA Cities Lawyers?
ReplyDeleteMaybe it's coming in the finale, but what happens if the emission spectrum of the surface were warmer and more centered around the N2/CO2 asym strech? Does it make those modes more effective? At Venus temps the spectrum would be more closely centered around these wavenumbers (if my math is right). Or does the reduced probability of there being excited molecules still leave it having a weak effect? (I have probability around .6% for each at 675K, again my math could be wrong)
ReplyDeleteWell, for one thing Venus has only small amounts of atmospheric O2 and N2 and the pressure at the surface will smear all the absorption spectra out. You are right that @ 750K the peak of the blackbody is at ~4 microns or 2500 cm-1, while @ 290 K it is ~ 12 microns or 800 cm-1.
ReplyDeletePheT has a nice blackbody spectrum app to play around with
https://phet.colorado.edu/sims/blackbody-spectrum/blackbody-spectrum_en.html
Just saw your newest post regarding pressure so will check it out.
DeleteYes Venus has a small amount of N2 but it is well over 400ppm. I somewhat understand your point regarding pressure, I'm sure the other article you posted will help me in that regard.
So in a few billion years when the sun is warming and expanding and earth hha reached a surface temp of 700K, does N2 then become an effective ghg?
In my paper (below) I explain blackbody radiation and emissivity are problems of thermoelectrics and not radiation - they are a correction factor, as all matter radiates at its identified and QM predicted spectra. Emissivity is really (I have deduced) the difference between the thermal electrical signal recieved from a sample and the real temperature of the sample. You mension ice here; ice has an emissivity of near 1, making it is a perfect absorber and emitter (NO WAY), we all know it reflects; and shiny metals by contrast have an emissivity such that they should not radiate (absorb or emit) at all (NO WAY), with E values of near 0. Something is wrong and I have attemped to answer it.
ReplyDeleteYour first figure of the IR specta and N2 O2 and CO2 is misleading and incomplete: you must include Raman Spectrocopies spectra as I have in my paper. Raman spectrometers measure temperature, very accurately, and through Plancks Law and Stefan Boltzmann Law, they show N2 and O2 radiate. A molecule of N2 in the thermosphere reaches temperatures (as measured by Raman Spec's) of some 2700K, and the air (N2 and O2) from a bolt of lightening, reached some 5 times the temerature of the surface of the sun - they absorb IR very well. You are all wrong.
https://www.academia.edu/36496299/Augmenting_19_th_Century_Thermoelectric_Greenhouse_Theory_with_20_th_Century_Quantum_Mechanics_Raman_Spectroscopy_Towards_a_Coherent_Radiation_Theory_of_the_Atmosphere
Why include Raman spectra? These are caused by inelastic scattering of highly intense UV radiation, not by absorption of IR radiation.
ReplyDeleteIR radiation *is* indeed absorbed by N2 and O2, but with extinction coefficients that are many magnitudes smaller than those of the greenhouse gases.
If you didn't know this, you better get back to first year spectroscopy classes. I'm sure the resident rabett can help with some useful textbooks.
Raman cross-sections are orders of magnitude smaller than quadrupole ones, roughly 10^-30 cm^2. Bunnies can forget that crap (FWIW look up DIAL LIDAR)
ReplyDeleteRaman spectrometers do measure, and with more accuracy than 'IR' (thermoelectric); IR spectroscopy is inferior to Raman, it is even used to calculate the Keeling curve, or has the potential to. (see my paper section 4.6.1). Raman measure temperature of an individual N2 molecule in space, where there is only radiation to an estimated 2700K !! . Look at my section 4.1.3 where I show H2O's mode is both IR- thermoelectric and Raman active. They are equivalent - only IR is imperfect, it does not send a good signal, this is what emissivity is.
ReplyDeleteAnswer this: without N2 absorption, how does an N2/CO2 laser operate? N2 is radiated, and it absorbs - right at its 2338 mode. GH theory say it does not absorb at that frequency. It does. Game over. N2 and O2 are not benign, they rule.
And these facts address Q3: the air absorbs the suns energy - directly! just as ocean/lake water does.
"without N2 absorption, how does an N2/CO2 laser operate?"
ReplyDeleteBlair does know that the N2 "absorbs" energy from elastic scattering of high energy electrons, yes?
"GH theory say it does not absorb at that frequency"
Nope, that's not what GH theory says. Measurements show that N2 has a negigible absorption at the relevant frequencies as compared to CO2 and H2O. Fortunately so, or the atmosphere would be largely opaque.
As marco says, CO2 lasers are excited by electric discharges.
ReplyDelete"As marco says, CO2 lasers are excited by electric discharges"
ReplyDeleteElectrical discharge is electron discharge; radiation of electrons in a 'cathode' tube.
By the Frank-Hertz quantum experiment, it is shown gas molecules absorb and emit this radiation in these tubes.
Electron radiation is assumed to be equivalent to photon radiation. They are both quantum 'particle' entities, as far as I understand.
To support this CO2 lasers can operate from either photon or electron excitation it has been shown.
Macro. If "N2 has a negligible absorption at the relevant frequencies as compared to CO2 and H2O" ; then this is not how it is demonstrated with this application and let's not forget N2 is especially 'long lasting' metastable.
Read my paper; especially the sections on thermoelectrics. I dare you to wire yourself up to a thermopile and amplify the signnal and then point it from the (safe) blue sky to a cloud. You'll fry. Water is thermo - electric: it generates an emf from thermoelectric transducers - as is CO2 CH4 O3 and glass and most solids, but not germanim.
Tyndall discovered the thermoelectric gases.
N2 and O2 are not. Nor is Ar (but it is not Raman either strangely)
Physics.
And let's not forget 'air', pertaining to collisions, is a near perfect thermal insulator / poor thermal conductor with a value of 0.024 (no units). This only really leaves radiation as a rational means of heat transfer; but this means is assumed nil with GH theory.
ReplyDeleteBlair, you are literally not making sense. You think it is strange Argon is not Raman active???
ReplyDeleteMacro: you just lost, and so did Rabett. If you cannot tell me where I am wrong and resort only to fallacy, you have lost. By Ar being 'strange' I was thinking in terms of its IR radiation signal. It does though have emission spectra lines, and it does get hot with excitation. Remember all matter radiates, by law; but Argon does not reveal this (strangely to me) by Raman spectroscopy or thermoelectric (IR). So it needs another spectrometer, and I don't know what that is. I look forward to finding it.
ReplyDeleteBlair, you seem to be blissfully unaware of your own lack of knowledge. *Of course* Argon does not show any signals in the IR region (neither observable by IR or Raman spectroscopy). It is monoatomic and thus does not have any vibrational motions (I know, I know, you *can* go to some extreme conditions where you get Argon to form compounds, e.g. the diatom, but those are extreme conditions)! Yes, you can excite it - excite electrons, that is, not any vibrorotational motions.
ReplyDeleteMacro and Rabett,
ReplyDeleteSeems like we are all good with Ar; now enough with the red herring and strawman, reply to my claims.
I wish to draw your attention to your first figure on this page ( you show name your figures) where the red IR emission spectra is shown along with the modes (that do not register by IR) of N2 and O2 and CO2s 2349. You seem to think that because the red curve is above the x-axis at the respective wavenumbers, they absorb some IR proportional to where the curve lies. This is so wrong and reckless; these modes do not show by IR, at all!! They are not IR active and are only distinguished by known (not to you?) selection rules. You have done Ad hoc science to make it look like these modes absorb IR as part of the 'standard model of GH theory.
As I have shown, N2 and O2 do absorb, at these QM predicted modes, but they are only shown by Raman technology.
To prove my point again, look at figure 1 of the following paper on green tech thermoelectric generators TEGs : it shows the output of a TEG. Notice output is the same shape as the atmospheric blackbody emission curve. I is not a coincidence, it is because the curves are the same; they are both products of thermoelectrics. They are both emf output. They are both produced by Seebeck effect thermoelectric transducers.
You are not the only ones who have it wrong; all of science has, they have right from the beginning of GH theory, and it looks like blackbody radiation theory is incomplete also; and no sceptic enjoys my discoveries - so I am alone. But I am right, and in time GH theory will collapse on what I have found. And I will update blackbody theory - emissivity will go.
I am going to update my paper with your first diagram to show how bad things have got, and include the paper I added here as I have not done that, I only found that today. What a prize.
Show me where I am wrong.
B
Blair, if you don't even understand why Argon is not IR nor Raman active, you don't deserve any response other than derision regarding your claims.
ReplyDeleteThe Rabett in *no* way implies that the indicated non-IR active modes of O2, N2 and CO2 do indeed absorb IR, but the Rabett rather uses a common educational tool to explain why there is no need to even consider these modes *even if they were IR active (as the asymmetric CO2 stretch actually is).
That N2 and O2 are Raman active is not a new observation, it's been known for many decades. What you fail to realize is the enormous amount of energy needed to excite these vibrational modes. It doesn't work with radiation of the same energy, it only works by measuring the indirect 'excitation' of the vibrational levels through the little bit of radiation that is inelastically scattered. Argon doesn't have any vibrational levels - it thus makes complete sense it is IR and Raman-inactive. This is basic physics, but something you seem not to understand.
And to top things off, you then start to blather about "atmospheric blackbody curve". Figure 1 is not an "atmospheric blackbody curve". It's a blackbody curve for a material at 290 K, and the Rabett is so kind to model the earth, for argument's sake, as a blackbody emitter of 290 K.
The link to the paper you were apparently referring to is missing. However, it is yet another weird argumentation, because TEGs convert *thermal energy* into *electricity*. They are supposed to be heated by an external heat source, and thus will heat up themselves and then start to radiate. However, this blackbody radiation should be reduced as much as possible in order to improve the conversion into electricity!
The Dunning-Kruger is really strong in you!
Macro,
ReplyDeleteThe papers again:
1) https://link.springer.com/article/10.1186/s40807-015-0016-y
2) https://www.researchgate.net/publication/234863498_Photovoltaic-thermoelectric_hybrid_systems_A_general_optimization_methodology
On your Dunning-Kruger claim, possibly, but equally, right back at ya. Almost an ad hominem, but not; I respect that possibility. Nothing has put me off my investigation yet.
The Ptolemy is strong in you (with respect to only geocentric); pinning everything on CO2.
How is that solar powered CO2 laser going? Is it working without N2?
And have you wired yourself up to my thermopile?
B
Blair, no problem getting a CO2 laser to work without nitrogen. It's output is a bit weaker, though.
ReplyDeleteNow, that supposed "solar-powered" CO2 laser is a bit tricky, as my impression is that you think the solar energy is used to directly excite the nitrogen. To the best of my knowledge, for a CO2 laser the solar energy is used to pump the gas to supersonic speeds and thereby excite the nitrogen. That is an indirect method.
Regarding the papers you refer me to, you might want to read what they show. That "blackbody curve" that is shown in Figure 1 is the solar spectrum. It is *not* the output from the TEG. Depending on how efficiently it transforms the incoming solar radiation into electricity, it *will* of course also have a blackbody emission, just significantly red-shifted.
You really, really, really should consider whether you should get some basic physics courses to understand what is going on here. Again, the fact that you do not understand why Argon is not Raman active suggests a major gap in your understanding.
I can't help but think of this video, and how well it fits you:
https://www.youtube.com/watch?v=hROzmSWFqEQ
Eli took a shot at a solar powered CO2 laser in the early 1970s without much luck, but they have been demonstrated, if no where else, in the atmosphere of Mars
ReplyDeleteThank you so much for the video treat; I am newly familiar with the term Pathological science, I recently picked up on it from this lecture ( https://youtu.be/7mGSVsl-ing?t=86 at 1:26). Given my hypothesis, and the response to it, disapproval from both sides, (yes so-called 'deniers' hate what I have) I don't think I am the pathological one - not until someone shows me where I am wrong; then, and only then, if I continue on anywaym that would be pathological. I'm just doing science.
ReplyDeleteOn: "figure is *not* the output from the TEG" ; it sure is, but yes it does appear to be a schematic. By first principles that is how a black body curve is produced, by thermo-electric transducers, all of them, they are all of the same kin as the original thermo-pile.
In support of my claim, now look at this paper ( file:///Users/blma/Downloads/Photovoltaic-thermoelectric_hybrid_systems_A_gener.pdf) , figures 1 and mostly 2. This paper is on the 'green tech' PVTEGs and their (pretty poor) efficiency, and it maps their output efficiency (fig 2). Notice the shape is the same as the black body spectrum, that's because, as I claim, they are the same. They are the same.
Emf production at say 1200nm will not happen unless the temperature rises, and even then it is poor.
Rabett and macro
On the solar-powered CO2 laser:
Before I go there, you have not acknowledged N2 absorbs electrons and photons at all at its 2338. And what about the molecular temp of N2 in the thermosphere. If one was to measure them by TE IR spectroscopy they would think they have no temperature (or are transparent!!!), but Raman spectrometers accurately measure them at around 2700K. Go figure!
So here is a paper that exploits this fact. It it also features in my paper at and around 4.7.2.
https://ntrs.nasa.gov/search.jsp?R=19840023547
“One laser concept that may achieve such efficiencies is the blackbody-pumped CO2 transfer laser. Such a system is called a fluid-mixing or transfer gas-dynamic laser and is shown in conceptual form in figure 1. In this system, a black body cavity is heated by collected sunlight (‘solar power’) to a temperature of approximately 2000 K. Nitrogen gas passing through the cavity is heated to the blackbody temperature. The vibrationally excited N2 then passes through a nozzle into a low-pressure laser cavity. Here CO2 and He are mixed with the vibrationally excited N2. A coincidence between N2 (v=1) (2338 cm- 1) and the OO1 asymmetric longitudinal mode (2349 cm-1) of CO2 allows rapid
transfer of the N2 (v = 1) vibrational energy into the CO2 001 upper laser level. Lasing commences between the OO1 and the 1OO levels at 10.6 microns. “
Busted!
Who's going to be my Rheticus?
B
Blair, I am afraid that even the average denier realises you are so far out of your depth you make no sense.
ReplyDeleteThat blackbody curve shown in Figure 1 is the *atmospheric spectrum* of incoming solar radiation. It's notably a calculated spectrum (look up AM1.5G). The second graph merely shows the conversion efficiency of photons into electricity. That these efficiency curves 'look like' the solar curve is logical: the materials used are chosen such that they are most efficient in the area where the sun emits most light.
And of course I am not going to acknowledge that N2 absorbs electrons or photons at "its 2338" (I assume you mean the 2338 cm-1 band). The extinction coefficient of electrons and photons with that amount of energy is extremely low. It can essentially only absorb *some* of the energy of much-higher energy photons and electrons that collide with N2. This is a bit easier to measure, but still not a very efficient process.
You have shown me we do have ourselves a problem; it is not with the climate.
ReplyDeleteYou just dismissed thermoelectrics and quantum mechanics.
"The second graph merely shows the conversion efficiency of photons into electricity": review the Seebeck effect and reflect on your words.
"I am not going to acknowledge that N2 absorbs electrons or photons..": review spectroscopy and quantum mechanics and reflect on your words.
Treat yourself with a view of Jupiter and its Galileon moons when you can, like I did last night, and preferably through a Galileon sized telescope, and reflect on what science is for you and what you want out of it.
Oh, and take another look at the definition of pathological science.
You have not told me where I am wrong.
I have answered questions 2 and 3, the others are redundant now.
Thank you for your review.
Blair
Blair, you cut out the important part of the sentence. Why? Dishonesty? Or because you don't understand quantum mechanics and firmly believe that Raman spectroscopy of N2 involves direct absorption of radiation of 2338 cm-1 energy?
ReplyDeleteThe very weak quadrupole absorption of N2 is at 2388 cm-1 (see above and part 1). Raman scattering takes place at a frequency DIFFERENCE between the illuminating light and the Stokes frequency (2388 cm-1 for N2) (hv-hvS) and is about as strong as the weak quadrupole absorption.
ReplyDeleteHello,
ReplyDeleteFor the record: I have updated my work after this dialog, and renamed it "Response to Judge Alsup’s Question Number 2". Thank you for your help. https://www.academia.edu/36496299/Response_to_Judge_Alsup_s_Question_Number_2_Whats_the_molecular_reason_that_CO2_is_a_greenhouse_gas_unlike_oxygen_and_nitrogen_Augmenting_19th_Century_Thermoelectric_Greenhouse_Theory_with_20th_Century_Quantum_Mechanics_Raman_Spectroscopy_Towards_a_Coherent_Radiation_Theory_of_the_Atmosphere
I have in the last month put out 2 preprint papers.
ReplyDeleteQuantum Mechanics and Raman Spectroscopy Refute Greenhouse Theory
https://www.researchgate.net/publication/328927828_Quantum_Mechanics_and_Raman_Spectroscopy_Refute_Greenhouse_Theory
and..
The Greenhouse Gases and Infrared Radiation Misconceived by Thermoelectric Transducers
https://www.researchgate.net/publication/329311153_The_Greenhouse_Gases_and_Infrared_Radiation_Misconceived_by_Thermoelectric_Transducers
B