What amateurs lack as a group is perspective, an understanding of how everything fits together and a sense of proportion. Graduate training is designed to pass lore from advisors to students. You learn much about things that didn't work and therefore were never published [hey Prof. I have a great idea!...Well actually son, we did that back in 06 and wasted two years on it], whose papers to trust, and which to be suspicious of [Hey Prof. here's a great new paper!... Son, don't trust that clown.] In short the kind of local knowledge that allows one to cut through the published literature thicket.
But this lack makes amateurs prone to get caught in the traps that entangled the professionals' grandfathers, and it can be difficult to disabuse them of their discoveries. Especially problematical are those who want science to validate preconceived political notions, and those willing to believe they are Einstein and the professionals are fools. Put these two types together and you get a witches brew of ignorance and attitude.
Unfortuantely climate science is as sugar to flies for those types. Wm Connolley points to an essay triumphally highlighted by Warwick Hughes:
High CO2 in the 1940’s atmosphere, contrary to IPCC scienceSo, according to our motto, we went and RTFR. Here we will briefly summarize the paper and then point out why it is wrong, not only wrong, but a) the information Beck points to has been well known for a very long time b) the reasons for the earlier measurements being much higher than the current ones have been well known for at least 50 years and c) these problems were the original impetus for the Mauna Loa observatory (MLO) series. In other words, Beck is quite right about the measurement methods and quite wrong about their interpretation. Local knowledge can be very important.
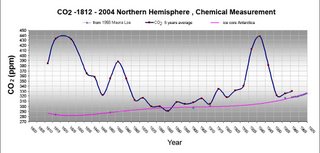
German biologist E-G Beck has assembled information from the scientific literature attesting to results of chemical determinations of atmospheric carbon dioxide by numerous scientists over the years 1820 to 1965. It is very interesting that many of these studies point to levels of carbon dioxide much higher than the IPCC preferred view of the evolution of carbon dioxide levels in the atmosphere. concentrations.''' Click here for a short discussion paper by E-G Beck.
Beck starts by noting that there have been a long series of measurements of the CO2 mixing ratio in air. He correctly points out that by 1900 various wet chemical methods were capable of measuring this to between 1 and 3% (3-6 ppm). He claims that
Callendar( engineer), Keeling (chemist) and IPCC do not evaluate these chemical methods though being standard in analytical chemistry, discredited these techniques and data and rejected most as faulty and highly inaccurate because not helpful proving their hypothesis of fuel burning induced rise of carbon dioxide in the atmosphere. In using their concept of unpolluted background level they had examined about 10% of available literature and considered <1%>But actually, Keeling was very familiar with the measurements, as can be seen in a letter he published in Science [This is in the JSTOR archive so it should be available to most (Science 102 (1978) 1109)], and a history of the MLO CO2 measurements (UPDATE: Link rot. Here is another link to the history) that he wrote ~1980.
Further, Beck is seriously overoptimistic about the accuracy and precision of the methods used before CD Keeling's introduction of the IR absorption method to quantify CO2 in the atmosphere. The best of the older methods in the 1950s could barely discern the seasonal cycle. Keeling's method, on the other hand did so easily with an accuracy of better than 1 ppm.
Click to enlarge
And if you measure in those rural spots that is what you get. You also observe a huge variabilty between measurements which is characteristic of situations where air pockets from urban areas are floating by. Because he is pushing this particular peanut up Warwick Hughes' hill, Beck misses probably the most important of CD Keeling's contributions. True, his spotting that CO2 mixing ratios could be much more accurately measured by IR absorption was major, but even more important was the long series of measurements he made during the 50s and 60s in California, the Western US, Hawaii and Antarctica which made absolutely clear that there were only a few places on earth where one could measure CO2 without local interferences. that also explains why most of the measuring stations in the current CO2 network are mid Pacific.
I am simply going to quote Keeling's Epilog which explains why Beck's analysis is wrong. For details, I refer the interested reader to the references at the end of Keeling's essay
UPDATE: Light editing added to kill off clunkers. Essentially this was written in the middle of a 24/7 experimental marathon. Apologies....... it seems paradoxical that truly reliable data were not obtained by investigators who deliberately sought undisturbed locations to obtain baseline CO2 data. As Bray (1959) noted, several nineteenth-century investigators, who claimed analytical analyses accurate to 1.0 ppm, made serious attempts to obtain data representative of locally undisturbed air. I conclude that these scientists, perhaps from an inadequate knowledge of meteorology and atmospheric motion, underestimated the difficulty in finding truly uncontaminated sites. When their analytical and sampling methods failed to give them the high reproducibility that they thought they had attained, they ascribed the scatter to the atmosphere itself and not to weaknesses in their methods.
In the first half of this century declining interest in atmospheric CO2 was kept alive by only a few investigators. The most notable was Kurt Buch of Finland, who concluded after many years of study that the CO2 concentration varied systematically with air mass. His claims (Keeling and Bacastow, 1977) that high arctic air had concentrations in the range of 150 to 230 ppm, north and middle Atlantic air, 310 to 345 ppm, and tropical air, 320 to 370 ppm, strongly influenced preparations for the IGY CO2 program, especially the Scandinavian program, which he initially supervised. When from inadequate chemical and sampling techniques the Scandinavian pre-lGY program produced CO2 concentrations in the same range as previous data, these new data were readily justified as resulting from different properties of the air masses passing over the sampling sites (Fonselius et al., 1956).
How long would the findings of the Scandinavian CO2 network have been accepted if new manometric and infrared studies had not been begun? The Scandinavian data continued to appear in the back pages of Tellus until after the infrared analyzer results for Mauna Loa and other locations had been presented at the International Union of Geodesy and Geophysics meeting in Helsinki in 1960. But reform was on the way. Walter Bischof in 1959 had assumed responsibility for Swedish measurements. He soon became suspicious of their variability on the basis of discrepancies between ground-level and aircraft sampling (Bischof, 1960). Also, he had begun to use an infrared gas analyzer. With this abandonment of the traditional chemical method of analysis, the Swedish CO2 data ceased to include unreasonably low CO2 values. Then in 1960 Bischof turned to investigating suspiciously high values using aircraft to verify ground-level data. Probably within a year or two, considerably more accurate systematic data would have begun to appear from the Scandinavian program.
But it is far from certain that a Scandinavian site as reliable as MLO would have soon been established. The Scandinavian investigators lacked the funds to embark on an ambitious continuous sampling program at a remote station. Many years might have passed before data of the quality of the Mauna Loa record would have been forthcoming. Indeed, high costs almost caused MLO to close down in 1964 in spite of its obvious value as a CO2 sampling site. Disruptions under that threat of closure account for a serious gap in the CO2 record during the early part of 1964. Problems of cost also contributed to the decision to shut down the South Pole continuous analyzer program at the end of 1963. If these two remarkable sites had not already been established and yielded high-quality data before 1964, it is likely that the stimulus to start work at such remote sites would not have occurred for at least several more years because of financial impediments. Thus it was a fortunate circumstance that Wexler and Revelle in 1956 saw the value of using the IGY organization to check out the possibility of near constancy in atmospheric CO2 by inaugurating a precise sampling program. We all recognize now that such a program is essential if we are to document adequately the rise in atmospheric CO2.
Peter, you can locate a fair amount of information about urban CO2 concentrations by using the search string - Co2 urban mixing ratio . One of my favorites, (because at one time I lived near a hot spot), describes measurements taken during trips through Essen Germany. You can also find information at the Ameriflux site, although it is not yet user friendly, and, of course, the data collection at cdiac .
ReplyDeleteThe problem with urban sites, and sites where pockets of air that are not well mixed can wander through, is that the measurments vary irregularly.
I´m amused to read a comment by an amateur who do not know the paper. It, s very interesting to read that the same pepople who had established modern knowledge of biology, medicine and nutrition science had obviously measured bullshit. So the basic relation of photosynthesis have to be corrected according to Keeling in a CO2/O2 relation of 1,2.
ReplyDeleteNice, a new science was born.
Anon, why don't you try that in German, it makes no sense in English
ReplyDeleteEli,
ReplyDeleteDo any systematic satellite based limb transmission (or other) studies of atmospheric CO2 exist? Can global measurements worth anything be done in such a way?
Eli - Graduate training is designed to pass lore from advisors to students...
ReplyDeleteDamn! No wonder my career never got off the ground. I wasted all my time trying to solve those damn problems.
Now they tell me!
Well, there are two answers to CIP:
ReplyDelete1. Serious: Think about how graduate training an apprenticships are congruent.
2. Truthful: Drinking a beer with your advisor gets you a hell of a lot further than sitting in your room solving problems.
You can see CO2 overtones in any solar limb spectrum (also down on the ground). Use [CO2 solar spectrum absorption] on Google to toss up a bunch of examples. It does you no good to look 1t 14 microns in the IR as the emission spectra are too bright
ReplyDeleteRe: the two links to Keeling. The first one requires a login and password, and the second link goes to a "404" message.
ReplyDeleteI'm impressed.
Thanks John, it was link rot. Eli has found another copy and added it as an update, but the search lead to something even more interesting.
ReplyDeleteBTW, a subscription to Science comes with AAAS membership. If you can't read it at your library or a nearby university, dues are about 120U$/year.
Agnotology
ReplyDelete"The availability of such large amounts of knowledge in this information age may not necessarily be producing a knowledgeable citizenry. Instead it may be allowing many people to cherry-pick information in blogs or news that reinforces their existing beliefs.[17] and to be distracted from new knowledge by repetitive or base entertainments...."
Got a lump? Don't ask 'auto insurance quotes', go see your doctor.
So as most of the CO2 emitted by humans dissolves in the ocean, am i right? and the absorbtion rate in the future is unsure due to saturation. Which will mean a higher concentration of carbon dioxide in our air. So why are we not doing a little more about the acidification of the worlds oceans to allow for more absorbtion, amateur hour here but just thinking??
ReplyDeleteKam,
ReplyDeleteThe dissolution of CO2 in the oceans is complicated. It depends not just on the chemical potential on either side of the water, but also on how the water mixes. That is one reason why it is so difficult to anticipate where the ocean will start being a source rather than a sink.
Can global measurements worth anything be done in such a way?
ReplyDeletePlease be much more specific about what you want to measure.
ReplyDelete