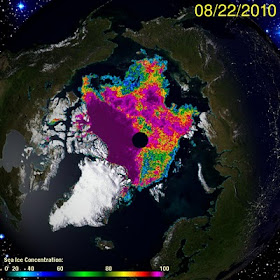
The east and west coasts of Greenland are ice free, the Northern Sea Route and the Northwest Passage Main Channel are open, and there is almost a route directly over the pole from the Beaufort Sea to the Greenland Sea with 50% ice cover or less.
 UPDATE: Anyone interested in Arctic weather can use the map at antropolis. Clicking on the location brings up a weather report. A nice toy, and today looks right toasty.
UPDATE: Anyone interested in Arctic weather can use the map at antropolis. Clicking on the location brings up a weather report. A nice toy, and today looks right toasty.The first tanker has cleared the Northern Sea Route and moved a shipment to China. In looking for this Eli found an interesting source of news about the European Arctic
Keep moving, never mind. Things are just as they were
There is joy in the world as PIOMAS (http://psc.apl.washington.edu/ArcticSeaiceVolume/images/BPIOMASIceVolumeAnomalyCurrent.png)shows the ice is reforming (???) at breakneck speed : )
ReplyDeleteHowever, my joy fades as I move from the novel to movie: http://psc.apl.washington.edu/zhang/IDAO/z_movie_heff_proj.mpg
Yeah that's all very well, but has the resident Wattamoron Goddard counted the pixels yet?
ReplyDeleteOr should we just believe our own lyin' eyes?
I wonder ... does the wily Rabett have a time machine he hasn't mentioned to us gentler bunnies? The figure seems to be from 10 years hence.
ReplyDeleteThe areas and tranches of broken and dispersed ice in the central pack are actually less marked now than they were a couple of weeks ago: weather (high pressures over the northern Beaufort Sea and then Greenland) has restarted the Transpolar Drift which has led to some compression especially around 165-180E.
ReplyDeleteAaron, that's the modeled volume anomaly, not the actual volume, that's "reforming". At this time of year, ice is still melting up there and the volume is still shrinking.
ReplyDeleteObviously the ice is recovering since there was less ice on 8/19 in 2007 than there is today.
ReplyDeletePenguindreams (Robert), you know full well that this is the figure from yesterday. But hey, it does look like something from 10 years in the future, just more ice by my reckoning.
I find it funny that the denialati can't see that the NW passage is open every year now when in the past it was never open. Strange.
dhogaza wrote, "Aaron, that's the modeled volume anomaly, not the actual volume, that's 'reforming'."
ReplyDeleteHow long until they might consider reentry into society?
Damn, RN, you mean Eli was taken by that nice sales lady when she sold him the way-forward machine?
ReplyDeleteWithout context, I have no idea what to make of this. Not all the bunnies are compulsive viewers of Arctic graphics.
ReplyDeletedamn it Eli, now I'm tempted to waste the day making movies on that xtranormal page
Ah! but what is fooling the model? I fear some of the ice has the density of a snow cone, floats high, and thus confuses PIOMAS. If so, then the color image above OVERSTATES the actual amount of ice.
ReplyDeleteAre we still looking for a hogshead?
ReplyDeleteRichard C
Eli,
ReplyDeleteA wayforward machine would be useful. Then we wouldn't have to depend on models to peer into a murky future!
Just remember what happened to the founder of Way Forward Technologies.
ReplyDelete(Clue: Douglas Adams)
The Northern Passage (catamaran piloted by two Norwegians) is halfway along the northern route sailing east, and about to strike out across the ESS for the Chukchi. Should find the NW Passage nice and toasty when they get there... Around the Arctic, both passages, single season, sailing boat. No, the ice is recovering nicely... ;-)
ReplyDeleteWhere does this image and data come from anyways?
ReplyDeleteRobert, look at the link. It is University of Illinois Champaign-Urbana. Cryosphere today is a well know site used as a source by both people who accept the science and those who deny the science (cough, cough, Steve Goddard).
ReplyDeleteFrom the Commodity Online article ("Russian gas reaches Asia via Northern Sea Route", 2010 Aug 17)...
ReplyDelete"Country's largest independent gas producer, Novatek, has completed its first tanker delivery of hydrocarbons via the Northern Sea Route to the Asia-Pacific region.
"According to Russia’s Transport Ministry, the trial journey was aimed at proving the economic feasibility of the northern alternative to the southern route lying through the pirate-infested Indian Ocean.
"Novatek said the debut delivery, started on August 14 from the Murmansk sea port, was made by a Sovcomflot Baltika oil tanker with deadweight of more than 100,000 tons and secured by Atomflot icebreakers."
I guess that is what you would call positive feedback.
My guess is that within the next 10 years they won't need an icebreaker escort.
ReplyDeletecarrot eater:
ReplyDelete"Without context, I have no idea what to make of this. Not all the bunnies are compulsive viewers of Arctic graphics."
Well, JAXA has us in a virtual tie with 2008 at the moment, i.e. a tie for 2nd place in the all time low extent category. The ice is quite spread out, and relatively thin, i.e. volume is the shits.
Check out this post at Neven's blog, which leads with a photo taken at 80N:
http://neven1.typepad.com/blog/2010/08/uscgc-healy-is-nearing-80n.html
You can see that with the right wind conditions, this would compact and extent would drop mightily. His second graphic shows the ship's position (the blinking red indicator doesn't contrast well with the purple ice extent coloration, you might need to stare for a moment, I did).
Good op-ed piece in today's NYT.
ReplyDelete"When it's spring time in Alaska its forty below" Johnny Horton Song
ReplyDeleteHe would not be mushing from Fort Barrow today, more than forty (F) above. Raining in Alert.
Rain is not good for ice?
Little Mouse
NYT op-ed said:
ReplyDelete"These experts are especially concerned that new patterns of air movement in the Arctic could disrupt the Northern Hemisphere’s jet streams — which are apparently weakening and moving northward. This could alter storm tracks, rainfall patterns and food production far to the south."
What do they think happened in Russia last summer? How often have you seen the jet stream do this? http://virga.sfsu.edu/pub/jetstream/jetstream_norhem/1007/10073100_jetstream_norhem.gif
Looks like a Black Swan event to me.
Eli, do you know anything about this event held earlier today at the ACS meeting? One would think that Christy's act is getting too long in the tooth to be respectable anymore even as a sop to fairness, but apparently not.
ReplyDelete.....I guess that is what you would call positive feedback.
ReplyDeleteYes indeed. And thanks to Eli for that link. Its the nuclear powered icebreakers which did it for me:)
Steve Bloom, my student went to that panel discussion. He said he was surprised at how many chemists didn't understand the basic physical chemistry of IR absorption of CO2. "They view the issue through their political lens rather than applying their scientific training" is how my student put it.
ReplyDeleteOn a brighter note, at least the Heartland Institute didn't have a booth at the exposition at the ACS meeting this fall.
Infrared absorption by CO2 isn't actually chemistry -- but I believe radiation transfer theory. However, they should know that it is possible to identify molecules by their absorption spectra I would presume -- and this would include of course CO2.
ReplyDeletehttp://www.globalwarmingart.com/wiki/File:Atmospheric_Transmission_png
... where the effects of atmospheric gases is shown on both incomeing and outgoing radiation.
*
By increasing CO2 concentrations, you increase the optical thickness of the atmosphere to thermal radiation. Increase the optical thickness of the atmosphere and you raise the effective height from which radiation tends to escape the atmosphere without being re-absorbed.
We can see the principle of optical thickness and how it applies to radiation absorbed by carbon dioxide here:
| This infrared image of the Earth was taken on
| 5 March 2005 after Rosetta’s closest approach
| to Earth by VIRTIS from a distance of 250 000
| kilometres and with a resolution of 62 kilometres
| per pixel.
|
| The image shows the distribution of CO2 bands in
| the Earth's atmosphere. In the green areas the
| CO2 concentration is enhanced.
|
| CO2 bands in Earth's atmosphere
| http://www.webcitation.org/5rgo0J0X0
| http://sci.esa.int/science-e/www/object/index.cfm?fobjectid=37190
... or if they prefer to see it in the lab:
| CO2 experiment: Iain Stewart demonstrates infrared radiation absorption by CO2
| http://www.youtube.com/watch?v=SeYfl45X1wo
Within certain spectral bands (as indicated by the spectra above) elevated levels of carbon dioxide locally increase the optical thickness of the atmosphere, implying that radiation escapes at a higher, colder altitude. This is what the satellite is measuring in terms of the spectra of thermal radiation then rendering in false color.
But lets say that we stop emitting carbon dioxide and let the system equilibriate. Once a new equilibrium is achieved the rate at which radiation leaves the system will once again equal the rate at which radiation enters the system. Neglecting changes in albedo, the brightness temperature of the earth as measured at a distance will return to what it was prior to the slug of carbon dioxide we put into the atmosphere.
But this brightness temperature will be the temperature of the atmosphere at a higher altitude. Now lapse rate is the rate at which temperature drops with increasing altitude and it is roughly constant in the troposphere. So given the lapse rate the Earth’s surface will be warmer — because we have increased the distance from the surface to the effective radiating altitude.
You might also want to check out...
effect of Temperature on CO2 absorption
WEDNESDAY, JULY 04, 2007
http://rabett.blogspot.com/2007/07/temperature-anonymice-gave-eli-new.html
It also has links to additional posts on the effects of pressure on the CO2 absorption spectra at the bottom.
Mark, mostly what chemists miss is that CO2 EMITS IR radiation as well as absorbs it.
ReplyDeleteThanks for the report, Mark. Ironically Christy wouldn't steer them wrong on that sort of issue.
ReplyDeleteIn other words, the chemists who have difficulty with the greenhouse effect are typically unfamiliar with Kirchoff's law -- which states that emissivity is equal to absorptivity, where spectral emissivity is equal to the ratio of emission for the material over the emission of a blackbody at the same temperature and frequency, and where spectral absorptivity is equal to the ratio of incident radiation at a given frequency that is absorbed by the material -- in this case the greenhouse gas.
ReplyDeleteIncidentally, one pitfall that people sometimes run into and that it is worthwhile to watch for is the view that by Kirchoff's law emission equals absorption -- but it doesn't -- and it in no way implies that the radiation field surrounding the object is at the same temperature as the object itself.
Under local thermodynamic equilibrium conditions (20 mb or above) a molecule will typically undergo a million or so collisions during the half-life of a the state of excitation. As such the energy of the photons absorbed by greenhouse gas molecules is almost inevitably thermalized (or "quelched") before it has a chance to be re-emitted as the result of spontaneous decay. As such the emission of thermal radiation is for all intents and purposes independent of the radiation field.
As the result of their temperature, greenhouse gas molecules will assume a Maxwellian distribution of molecule velocities, and given the collisions molecular energy will be distributed in accordance with the equipartition theorem. As such under local thermodynamic equilibrium conditions a certain percentage of greenhouse gas molecules will be in an excited state at any given moment. And re-emission is the result of the spontaneous decay of an excited state, it has no memory of how long a molecule has been in an excited state, and over a given period there will be a certain percentage of molecules which undergo decay by means of emission.
And as the result of the equipartition theorem the brightness temperature associated with each quantized state of molecular excitation will be the same as the others -- just as the temperature of each gas that makes up the atmosphere (locally) will be at the same temperature. And by this we mean the corresponding temperature that a blackbody would have to be for its brightness at the wavelength of the state of excitation times the spectral emissivity of the gas to be equal to the brightness of the gas at that wavelength.
*
A quick aside: re-emission is properly thought of as re-emission by the gas, not the molecule as the molecule that absorbs a photon is in all likelihood not the molecule that originally absorbed the photon. And as a matter of fact, the photon that gets emitted and energy that it represents is in no way the photon that was absorbed or the energy that it represents -- as through the process of thermalization the energy that the gas absorbed when a given molecule of that gas absorbed the photon is spread out by the large number of collisions until it is shared by a very large number of the molecules making up that gas.
(continued below)
(continued from above)
ReplyDeleteFinally, the phenomena of quelching brings us to another point where skeptical chemists might have difficulty with the greenhouse effect -- they may inadvertently think of the energy acquired by greenhouse gases belongs simply to the gas itself rather than being shared by all the gases that constitute the atmosphere. This is a point that Eli has raised before:
"Any person, particularly a skeptical chemist, would expect that, with the nonstop emission of thermoradiation from Earth's surface, all CO2 molecules would soon be in the excited vibrational and rotational levels of their molecular energy states, and none would be left to absorb more outgoing energy. Hence, the greenhouse effect would be very limited.
"However, CO2 molecules do not exist alone in the atmosphere. The excited molecules can and do transfer their excess energy to other molecules and return to ground states and are therefore ready to absorb thermoradiation again. The transfer of the initially absorbed energy to other nonabsorbing molecules, called 'quenching' in photochemistry, enables a relatively small amount of greenhouse gases such as CO2 to continuously absorb the thermoradiative energy, which otherwise would escape into space, and to convert the radiation back to thermal energy that stays on Earth."
Getting Rudy's Back
Eli Rabett, AUGUST 31, 2009
http://rabett.blogspot.com/2009/08/getting-rudys-back-next-latest-issue-of.html
Very clearly put, Tim, and thanks. But is it "quelching" or "quenching"?
ReplyDeleteSteve Bloom wrote, "Very clearly put, Tim, and thanks."
ReplyDeleteThank you! The last two comments were all from memory. I wanted to see how well I could do without referring to anything.
Although late last fall I had gotten the bit about "... it in no way implies that the radiation field surrounding the object is at the same temperature as the object itself" wrong. I had more or less stated at one point that the radiation field and object (or atmosphere) had to be locally in equilibrium in order to meet the condition of local thermodynamic equilibrium.
I was wrong, Rod B. caught me on it at Real Climate -- and I thanked him for pointing it out to me. Afterwards someone started arguing with him, getting confused and taking Kirchoff's law to mean that emission is equal to absorption. I argued at some length with them while commending Rod for being for pointing out what Local Thermodynamic Equilibrium actually means.
I am proud of getting things right. But I take greater pride in my ability to admit when I am wrong.
*
Steve Bloom wrote, "But is it 'quelching' or 'quenching'?"
"Quenching" I believe as this is how Eli refers to it -- and he is the one I got it from so I figure he's right.
"Infrared absorption by CO2 isn't actually chemistry", I was ready to go on a rant of why historically and practically it is chemistry, but I'll settle for "Oh, purleaze!".
ReplyDeleteRichard C
"'Infrared absorption by CO2 isn't actually chemistry', I was ready to go on a rant of why historically and practically it is chemistry, but I'll settle for 'Oh, purleaze!'."
ReplyDeleteThank you, Richard. That was a real treat. Rarely have I seen such a convincing argument delivered with such eloquence and brevity.
But perhaps you could still grace us with a link? Thanks again.
Tim's explanation is indeed excellent. The thing with chemists is that they are used to taking IR absorption spectra in which the source is collimated IR from a heated blackbody held at ~500 C. In such conditions you never see the diffuse emission from the gas/liquid/solid in the cell, so they don't believe it. Besides, most of them are organikers.
ReplyDeleteSpeaking as a chemistry and materials science graduate, lab rat, and now something of a bit of experimental arcaheologist etc, I thought that chemistry was principally involved in reactions between atoms, and hence stuff to do with absorption etc of IR is on the boundary between chemistry and physics. It has been used by chemists for a century or two, but the actual explanation of how it works is in the realm of physics. So I would say using an IR spectroscope is part of chemistry, but not necessarily using chemistry, if you see what I mean.
ReplyDelete(MInd you I'm not into organic stuff, I prefer inorganic and ceramics and suchlike)
This comment has been removed by the author.
ReplyDeleteguthrie,
ReplyDeleteSo this would be comparable to a virologist using an electron microscope to study viruses even though virology doesn't actually include electron microscopes as part of its subject matter. Or to consider something much more basic, when I am looking at a rock it is the rock that I see, not my eyes, even though my eyes are the specific means by which I see the rock.
Actually the example of how knowledge in virology is at least partly dependent upon our knowledge of the physics that underlies electron microscopes would be illustratative Duhem's Thesis regarding the interdependence between different scientific theories and even branches of science that makes strict falsifiability inapplicable to modern scientific theories -- even though such theories remain testable.
A Question of Meaning : Part [9]: Foundations, Coherence and the Principle of Falsifiability : Section 3: The Refutation of Karl Popper
;-)
Pretty much, yes. And I've never seen science as being anything other than interrelated, since probably before I went to university. (Although they should have taught us philosophy of science as well)
ReplyDeleteWhich is where the denialists, with their one track minds, cannot cope, and end up sounding like creationists.
Ummm reminder guys that there are plenty of physical chemists who take no issues with any of these matters.
ReplyDeleteAnd ummm: http://web.uct.ac.za/depts/mmi/stannard/linda.html
Other than that quite a good discussion. I prefer to read than post here for the most part as I like Eli's thorough and funny posts, and Tim did a good job indeed, however, virology is more diverse than you indicate and P-chem is very real physica and chem combined.
Tim, I posted a little too hastily so I now see what you mean in terms of EM, so my immediate apologies there.
ReplyDeleteConnections, Part I of III
ReplyDeleteguthrie said,
"... I've never seen science as being anything other than interrelated, since probably before I went to university. (Although they should have taught us philosophy of science as well)
"Which is where the denialists, with their one track minds, cannot cope, and end up sounding like creationists."
The interrelatedness of all scientific knowledge forms the basis for an analysis of creationism that I made a while back. It was in a paper where I argued that creationism results in the epistemic and ethical corruption of the minds of its adherents. I intended for religious leaders.
In it I stated:
"A religious view rooted in science will be grounded in the shifting sands of scientific discourse, placed in constant threat of being uprooted by the newest scientific discoveries. For the better among those who initially accept this substitute for true faith, such a view will at first seem intoxicating, but will soon prove poisonous to their religious beliefs.
"For others, the proper religious stance becomes transformed, and the proper intellectual courage to revise one's beliefs when confronted with new evidence is transmuted into its polar opposite. Intellectual 'courage' becomes the will and the power to challenge, doubt and deny any body of empirical evidence or knowledge whenever it comes into conflict with their religious or political beliefs. At this point, one of the most fundamental ethical virtues -- honesty -- has itself become undermined, and with it all the virtues which would normally be encouraged and taught through the moral guidance of religion. Properly, religious leaders who understand what is at stake will oppose 'empirical' faith both for the contradiction which it embodies and as the antithesis of the true faith they seek to protect and nourish."
Religion and science
http://www.bcseweb.org.uk/index.php/Main/ReligionAndScience
Essentially, a very large part of what science relies upon is that given different lines of investigation. Over time a conclusion that becomes well-established will accumulate multiple lines of justification, and the justification that it receives from those lines will tend to be far greater than that which it receives from any one line of investigation considered in isolation from the rest.
As long as a given thread of justification is considered in isolation from others the justification for that thread is typically something that can be easily denied -- by denying only a few other things. But over time if one wishes to continue to deny a given scientific conclusion it becomes necessary to deny more and more of the world that science illuminates for us.
Connections, Part II of III
ReplyDeleteThis likewise reminds me of a passage I had written that expressed a thought normally attributed to Hegel ("The True is the Whole") but which is probably much older and I framed in terms of science:
"Empirical science itself is a unity because reality is a unity. There exists degrees of justification, but greater and lesser degrees of justification exist throughout all of empirical science, particularly if there is any area of active study, and no one branch is truly privileged over another. There necessarily exists a cognitive division of labor due to the limitations of individual human awareness, but the criteria employed for establishing the permeable boundaries between disciplines are ultimately pragmatic in nature."
I had lost track of that passage -- but oddly enough it is in one version of an essay that I linked to over at Deep Climate's here just yesterday. But not the same version. I am going to have to merge the branches, I think.
Connections, Part III
ReplyDeleteI once somewhat humorously expressed much the same idea along these lines:
"I suppose you think that the likelihood that a given scientific conclusion is wrong decreases as an exponential function of the number of lines of evidence.
"That only works for members of the reality-based community. For denialists its the other way around. Knock out any one line of evidence and you've knocked out the conclusion — at least until someone else brings up the other lines of evidence. But then you can ignore them, go home, come back tomorrow and start afresh."
Comment 277 of Advice for a Young Blogger, 18 March 2009 at 2:19 PM
*
Fortunately for me one of my philosophy of science professors actually did deal with it -- to a degree. His name was Evan Fales, and I believe it was back in 1991. He didn't actually mention the name Pierre Duhem -- who arrived at the thesis back in 1892. Regardless, that day it was like lightning had illuminated the sky. And that day -- that presentation -- is what inspired "A Question of Meaning" -- something I wrote only years later.
Jacob -- no problem. Incidentally, I am fascinated by viruses as well.
ReplyDeleteHere is something that you might like, but please don't link to the blog or essay. The blog may be going bye-bye and the essay is a rough-draft that needs references and to be expanded.
Please see:
Retroelements in Evolution
Posted on 2010/08/27 by Timothy Chase
http://timothychase.wordpress.com/2010/08/27/retroelements-in-evolution/
Tim, I like your blog and I added some comments even if the location is ephemeral.
ReplyDeleteTo reiterate P-chem is all about radiative transfer and what not:) Keep up the good work on introns and alternative splicing mechanisms.
Tim
ReplyDeleteI not sure how to grace you with a link to my degree!
Chemistry is the study of chemicals: Their properties, reactions and interactions, no?
Richard C
Richard C, chemistry is the study of matter, its transformations, and the energy changes associated with those transformations. Understanding how molecules gain and lose energy (via electromagnetic radiation and collisions as Tim points out) is certainly in the realm of chemistry. That is not to say that it is of interest to all chemists. (As Eli points out, the organikers in particular)
ReplyDeleteIn an attempt to drag this thread back on-topic, please note that as of today JAXA's sea ice extent number has dropped below Steven Goddard's predicted 5.5 million km^2.
ReplyDelete(assuming one lets him off the hook for his earlier "recovery to 2006" "prediction".)
Richard, the way I view it is that while photochemistry is certainly a branch of chemistry and for example high energy photons are involved in chemical reactions in the stratosphere during the splitting of the ozone molecule -- making it photochemistry, no actual transformation of a chemical from one to another is taking place when carbon dioxide absorbs or emits infrared radiation. Therefore at that point while you have the interaction between radiation and matter it isn't chemistry, not even photochemistry.
ReplyDeleteThe absorption and emission of thermal radiation by carbon dioxide is still physics, radiation transfer theory and quantum mechanics as it involves the interaction between radiation and matter. But for it to be chemistry you would have to have the transformation of one set of chemicals into another -- similar to the splitting of ozone in stratospheric chemistry. No such changes are occurring. Therefore it isn't photochemistry. Of course it is possible that I am missing something.
dhogaza, Yesterday I saw a large region go to blue -- and I expected that region to disappear the next day. Sure enough. A large region -- and the ice is just gone.
ReplyDeletePeople can see it here:
http://arctic.atmos.uiuc.edu/CT/animate.arctic.color.0.html
Left-click on the squares representing the frames that you don't care to see so that they change to red and slide to the left the scrollbar in the upper right so that you can watch the changes from one frame to another.
Regarding the question of whether the absorption of infrared radiation by carbon dioxide counts as either chemistry or photochemistry, Richard wrote, "Chemistry is the study of chemicals: Their properties, reactions and interactions, no?"
ReplyDeleteI think I can see what you are arguing here: your are taking the absorption spectra of a molecule to be a property of the molecule itself. With me I am viewing the interaction between the infrared light and greenhouse gas as an interaction, as something relational, and therefore not as a property of the molecule or gas.
It is the difference between intrinsic properties and dispositional properties where presumably the dispositional properties would derive from the intrinsic properties of the objects themselves. I am thinking in terms of intrinsic properties and you in terms of both intrinsic and dispositional properties.
However we cannot know the intrinsic properties of a thing except insofar as it interacts with and therefore relates to something else. This being the case one could easily argue that the dichotomy between intrinsic and dispositional properties is artificial.
So you may have a point.
Personally I would argue that the difference between intrinsic and dispositional properties is epistemological, that is, a matter of context. Thus it makes sense to regard the absorption spectra of carbon dioxide a (dispositional) property of carbon dioxide. But when you are simply speaking of the absorption and emission of radiation by carbon dioxide this wouldn't be a property of carbon dioxide itself but a relationship between carbon dioxide and radiation.
The difference being whether or not anything is gained -- that is in terms of cognitive economy -- by bringing in a dispositional property. Furthermore there is most certainly an "intrinsic" property of sorts that is readily identifiable in the case of carbon dioxide absorbing and emitting thermal radiation: quantized state of molecular excitation.
But is the change from one state to another and back a change from one category of molecule to another? I would argue that it isn't -- based on the reversibility of the transformation -- as it applies to the molecule itself.
dhogaza, earlier in the thread you had written, "You can see that with the right wind conditions, this would compact and extent would drop mightily. His second graphic shows the ship's position (the blinking red indicator doesn't contrast well with the purple ice extent coloration, you might need to stare for a moment, I did)."
ReplyDeleteNow I have just observed, "Yesterday I saw a large region go to blue -- and I expected that region to disappear the next day. Sure enough. A large region -- and the ice is just gone."
What you were suggesting was a somewhat superficial change: the compactification of ice by the winds -- but not the actual melting. The idea being that the extent greatly inflates the apparent amount of ice.
However it would appear that the change that I have just observed isn't due to compactification. If it were you wouldn't expect to see the distance that the boundary has traveled to be so great.
The extent changing as the result of a long stretch of ice with the boundary moving a short distance perpendicular to the length of the ice? That could be due to compactification.
But in this case the boundary moved a fairly large distance overnight. Much faster than the merely circulation of the sea ice. It would appear to have been due to melt. The ice is thin.
"What you were suggesting was a somewhat superficial change: the compactification of ice by the winds -- but not the actual melting. The idea being that the extent greatly inflates the apparent amount of ice.
ReplyDeleteHowever it would appear that the change that I have just observed isn't due to compactification. If it were you wouldn't expect to see the distance that the boundary has traveled to be so great."
The fact that extent was inflated was based on both the wind patterns at the time (spreading ice out), and the fact that the extent/area ratio was higher than typical (people over at neven's blog were tracking that).
There's no doubt that compaction plays a role when wind conditions are right. This is not to say that the ice isn't melting. Also, obviously it was and is.
For the past few days (week? week+?), conditions have also been good for pushing ice through the Fram Strait. That should be melting as it moves into more southern waters but the immediate impact is that it tends to spread out and thus get caught as a local increase in extent, until it melts below that 15% threshold.
CT's area anomaly is between that of 2008 and 2009 at the moment, as is extent.
"But in this case the boundary moved a fairly large distance overnight. Much faster than the merely circulation of the sea ice. It would appear to have been due to melt. The ice is thin."
Apparently there's been some really warm water there (by ice standards). Thin ice melts faster. Ice that's spread out melts faster. Thin ice is more easily moved around by wind, causing extent to grow or shrink depending on conditions.
And ever-shrinking volume is the real story.
There are plenty of spectroscopists in chemistry and physics departments. So the absorption of infrared by CO2 is chemistry. Physical chemistry. The people who synthesize toad toxins (for example) may regard spectroscopy as physics, of course.
ReplyDeleteGerhard Herzberg was awarded the Nobel Prize in Chemistry, although he always thought of himself as a physicist. So if the mighty Herzberg is confused, what hope remains for the rest of us?
-Nevada Ned
Ahh, so Timothy Chase has been involved in the BCSE. So was I for a wee while a few years ago when the creationists were busy in Britain. Small world and all that.
ReplyDeletePhysical chemistry is the bit between chemistry and physics, I agree.
Compactification is a word? I thought the one to use was compaction?
guthrie wrote, "Ahh, so Timothy Chase has been involved in the BCSE. So was I for a wee while a few years ago when the creationists were busy in Britain. Small world and all that. "
ReplyDeleteYes, I actually knew some of the people from DebunkCreation and in late May 2006 got involved with a group that later formed the British Centre for Science Education two months before there was even any talk of BCSE. I was there for about a year. YEC David Anderson even personally wrote me into his great atheist conspiracy story as one of the conspirators. I took as a compliment -- coming from him.
And actually creationism is still quite a problem from what I can tell. And it would appear that creationism is getting taught during science lessons in some schools even today -- so I am not sure that things have really gotten any better. The creationist movement in Britain and Ireland is well-financed and has been receiving help from similar organizations in the US. And it may be much more difficult to stop there as the Separation of Church and State isn't exactly a well-established principle there.
*
guthrie wrote, "Compactification is a word? I thought the one to use was compaction?"
Actually "compaction" is undoubtedly more appropriate.
"Compactification" is something that you normally do only to spaces, rendering infinite dimensions finite or periodic. Even the word "compactify" normally gets employed that way -- although sometimes it will get used colloquially to mean "make compact" in a way that isn't necessarily limited to spaces.
But while I am a physics geek and may have picked up the term from there, "compactification" (in the sense that the term is "normally" used) isn't something that I would normally think about. And as for my mishandling of the term it was just "handy" at the time -- and as is too often the case, I was a bit rushed in getting things out. My apologies.
Guthrie -- did you come into the BCSE as part of the merger between Science Just Science and the British Centre for Science Education? I know there was a Guthrie from SJS and the name had sounded familiar.
ReplyDeleteSomewhat of topic, but Eli -- you've comment at DotEarth on this...
ReplyDeleteAndrew,
I seems that your complaints have to do with the press coverage. Your two featured links have to do with a press release and Huybers comment on the fact that the highest profile journals are looking for headline worthy studies. Yet it is well established that press releases often do not tell the whole story and they are not the scientific papers in question. Nature and Science also suffer from this affliction, yet it is not just in climate science, but in every other field in which they accept papers.
There are pluses and minuses to this approach. On the plus side, they bring to light important research which is worthy of further investigation. On the minus side they may publish marginal research which is worthy of further investigation. Notice something in common here? A good recent example of this (apparently in the former category) is the recent paper in Nature which found sharp reductions in phytoplankton populations worldwide. I read the preprint and it seems like good work, and is in line with other recent (less high profile) work on the causes of phytoplankton blooms (work which had been published the month before). Seems good, but it needs more investigation.
In your post about the NSF press release you cite Stephen Schneider who says that the results of the Pounds paper are provisional, but you criticize the IPCC for using the Pound paper when there had been no published research contradicting those findings. That did not come until 2008. So I assume that you think the IPCC should have a “way forward machine” so that they can see the future critiques of research they cite?
You never did talk about the difference between climate science and cognitive science. My take on the Hauser problem is that he succumbed to confirmation bias in interpreting data which he gathered. Once he did release his videos and others could look at the videos they found no evidence of what he claimed to have observed in monkeys. Did he falsify his data or just misinterpret it? It was hard to tell from the press reports (including those in the NYT) why Harvard came to the conclusion they came to. No links to the primary documents, etc. You should probably make your points (or lack of them) more explicit. Just how do Hauser's problems relate to climate science (as practiced, and not in press releases) relate to each other? Offhand, I can't see the relation. For most datasets, climate scientists (at least those looking at changes) have full access to the data used by people doing the research and can do their own analysis. There is little room for interpretation of the raw data, interpretation is limited to how the data is analyzed.
Oh yes, SJS, that'll be me then. I'd almost forgotten about that. It was a few years ago and interesting things have happened since then. I was more on the fringes, doing letters to the editor in my local paper, MP's and some forum arguing, as well as letting textbook publishers know that creationists were slagging off or misusing their texts. I see the new gvt's policies have creationists salivating with glee, so I might have to send off a few letters. (I hung out at AtBC for a while as well, I see it hasn't changed much in 4 years)
ReplyDeleteI take it I'm not the only person a little concerned by the amount of open water? Not that I know what will happen, but the amount of melting seems ominous.
Tim,
ReplyDeleteI think you see, what I see in the ice. That is; ice that has significant voids in it so that it floats high and fools the volume measurements, but is very weak, so when a swell hits it, it breaks and tips over, leaving nothing but a skim of tiny ice pellets on the surface of the water.
Of course, if we are failing to sense the melting of voids in sea ice, we are likely also failing detect the melting of voids in land ice.
I do not think one can do chemistry without physics, or (real world) physics without chemistry. Other than for union work rules, why would we need to draw a line between chemistry and physics? Everyone should know both.
JAXA: down to 5.4 million km^2, and no sign of a significant let-up that I can see. That scattered stuff north of Alaska seems to continue to be disappearing quickly, while ice appears to still be getting pushed out of the basin through the Fram Strait.
ReplyDeleteIt will end up below 2009 but above 2008, I do believe, my guess all along (and it was just a guess), but there's a lot of spread out, thin ice compared to 2008, IMO.
This:
ReplyDeletehttp://polar.ncep.noaa.gov/sst/ophi/color_anomaly_NPS_ophi0.png
Shows a lot of water above 0C, and if this keeps up, quite a bit of that ice north of eastern siberia should melt out, I'd think ...
Oh, sorry, that's a map of temp anomaly, not temp ... still, I'll go with "warmer than usual" being favorable to continued melting ...
ReplyDeleteAaron wrote, "I think you see, what I see in the ice. That is; ice that has significant voids in it so that it floats high and fools the volume measurements, but is very weak, so when a swell hits it, it breaks and tips over, leaving nothing but a skim of tiny ice pellets on the surface of the water."
ReplyDeletePossible. I am still inclined more to melt given the pattern, but as my misuse of the term "compactification" would suggest I am not expert in this area.
*
Aaron wrote, "I do not think one can do chemistry without physics, or (real world) physics without chemistry. Other than for union work rules, why would we need to draw a line between chemistry and physics?"
At first the answer seemed obvious to me -- but I found it interesting that others found the opposite answer obvious. So it was largely a matter of curiosity -- and the problem became interesting in its own right, for example, in how the "dichotomy" between intrinsic and dispositional properties might apply.
*
"Union work rules"? In a sense.
There is obviously a degree of interdependence and overlap between the two domains. But if for example one can distinguish be one domain and the other then you can use it to illustrate the interdependence between two branches. But even in the presence of such interdependence it is possible to distinguish between one domain and another.
*
And while there clearly is a sense in which materially all chemistry ultimately reduces to physics -- I am thinking quantum mechanics -- there is also value in treating it as its own area of specialization. Just think of how difficult it would be to try and treat chemical reactions as things to be solved in terms of quantum mechanics.
Although it is my understanding that we have been able to derive the behavior of water, including its bulk properties, phases and transitions in terms of quantum mechanics using numerical methods, the task would be monumental in the case of most of the reactions studied by chemists. Besides, physicists would simply take over and chemists would be out of work.
*
And at a more theoretical level -- largely as a matter of habit -- I am pretty much always interested in the distinction between the object of awareness and the means of awareness. The rock that I see and the eye with which I see it.
This distinction always helps in terms of identifying the hierarchical structure of knowledge. Thus in showing how the knowledge claims made by science are different from those made by the religious fundamentalist or the relativist.
The distinction between the how and the what of awareness and knowledge permits one to respond to the criticisms made by extreme skepticism that otherwise opens the door to both. But the last is more of a theoretical concern given the context of our discussion.
*
More importantly, nowadays the interdependence between different branches of science is requiring more interdisciplinary teams and collaboration. And by distinguishing between the different branches it becomes easier for members of the team belonging to one discipline to see the need for involving members of different disciplines. It helps one clarify how the disciplines are related so as to better coordinate the work. So while "union work rules" was meant in jest, there actually is some truth to it.
*
Aaron wrote, "Everyone should know both."
I would most certainly agree. Especially on collaborative interdisciplinary teams.
Anyway, don't mean to reopen the discussion regarding the specifics. Not sure there would be much to gain from at this point. Did want to clarify the nature of my interest in the topic, though.
Climate Progress has a new post up on the Arctic, how we are headed towards a new record low, how the Northwest and Northeast passages have opened up and even how the Medieval Warm Period appears not to have extended to the Arctic - judging from sedimentary proxies. Includes pictures, charts and links.
ReplyDeletePlease see:
Arctic sea ice volume heads toward record low as Northwest Passage melts free fourth year in a row
http://climateprogress.org/2010/08/28/arctic-sea-ice-volume-northwest-passage-david-barber-antarctic-sea-ice/
Also, for those of you that may not be aware of Neven's Arctic Sea Ice (a blog) or who haven't visited it any later than Aug 24, there are three Aug 25 posts well worth checking out -- including photo animations showing the sea ice flow/transport including what at least appears to be shelf breakup along Greenland's east coast. However, while the breakup looks quite dramatic, however, Neven did some research and it is apparently just business as usual for the coast at this time of the year. The ice there will reform -- this winter.
Please see:
http://neven1.typepad.com/blog/
Does the penetration of the swell throughout the ice pack count as a tipping point? It didn't used to happen.
ReplyDeleteWe have a new type of Arctic Anomaly and now a new type of El Nino.
The times they are a chang'in. The first peek at a new paradigm?
Little Mouse
Little Mouse wrote, "We have a new type of Arctic Anomaly..."
ReplyDeleteNow called the Arctic Dipole but called the Arctic Rapid change Pattern (ARP) here:
Xiangdong Zhang et al. (2008) Recent radical shifts of atmospheric circulations and rapid changes in Arctic climate system, Geophysical Research Letters, Vol 35, L22701, doi: 10.1029/2008GL035607http://folk.uib.no/gbsag/Zhang_etal_2008.pdf
Little Mouse wrote, "... and now a new type of El Nino."
Sang-Wook Yeh et al.(24 Sept. 2009) El Nino in a changing climate, Nature, Vol 461, pp. 511-5
http://www.environmentportal.in/files/El%20Nin%CB%9Co%20in%20a%20changing%20climate.pdf
Karumuri Ashok and Toshio Yamagata (24 Sept. 2009) The El Nino with a difference, , Nature, Vol 461, pp. 481-4
http://www.indiaenvironmentportal.org.in/files/The%2520El%2520Nin%2520with%2520a%2520difference.pdf
Little Mouse wrote, "The times they are a chang'in. The first peek at a new paradigm?"
So it would appear.
Oh, yes, it's a new paradigm, that's why sea ice volume is so important, after all ...
ReplyDelete"Does the penetration of the swell throughout the ice pack count as a tipping point? It didn't used to happen."
makes clear that extent doesn't fully capture what's happening. Area measures are better (well, would be, if the satellite sensors didn't lead to confusion between melt ponds and open water), but obviously volume is best of all.
Anyway, JAXA has extent down to 5.45 ... it will probably be right around 5.4 tomorrow.
JAXA has been below the 2009 minimum for a couple of days now, and the three day extent loss has been the highest for any 3 day period within or partially within september in the 8 year JAXA record.
ReplyDelete(I've poached this from someone else's comment at Neven's blog.)
Bremen has current extent below the 2009 minimum also, and I suspect NSIDC, too, though I've not dug through the data.
So what will Goddard come up with now that the "arctic sea ice minimum has recovered for N years in a row" argument has collapsed?