 Carbon sequestration is one of those things that might be a good thing, and on the other hand might not. Roger Jr, and Andrew Dessler are duking it out at Nature Geoscience. Issues include the energy cost of sequestering the carbon, the seal on the storage volume and more. However, IEHO, sequestration is a poor second choice. Better to find something useful to do with the CO2, a point that has escaped everyone except those actually working on the problem. There are a variety of clever ideas, whose principal virtue is that if you can extract value, especially energy, from the CO2, you don't have to have an absolutely perfect sequesterization to have a real effect.
Carbon sequestration is one of those things that might be a good thing, and on the other hand might not. Roger Jr, and Andrew Dessler are duking it out at Nature Geoscience. Issues include the energy cost of sequestering the carbon, the seal on the storage volume and more. However, IEHO, sequestration is a poor second choice. Better to find something useful to do with the CO2, a point that has escaped everyone except those actually working on the problem. There are a variety of clever ideas, whose principal virtue is that if you can extract value, especially energy, from the CO2, you don't have to have an absolutely perfect sequesterization to have a real effect.Curtis Oldenburg at Lawrence Livermore has a potentially useful idea to combine carbon sequestration and enhanced gas recovery, using the CO2 to push methane out of old natural gas wells.
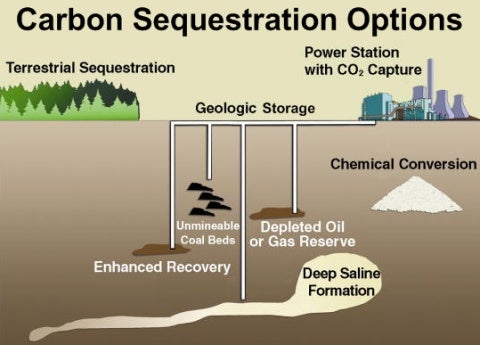
With their proven records of gas recovery, demonstrated integrity against gas leakage, existing infrastructure of wells and pipelines, and land use history of gas production and transportation, depleted natural gas fields are attractive targets for carbon sequestration by direct carbon dioxide (CO2) injection. The International Energy Agency (IEA) estimates that as much as 140 GtC could be sequestered in depleted natural gas reservoirs worldwide (IEA, 1997) and 10 to 25 GtC in the U.S. alone (Reichle et al., 1999). Although target gas reservoirs for carbon sequestration are depleted in methane (CH4) with pressures as low as 20–50 bars, they are not devoid of methane. Prior studies have suggested that additional methane can be recovered from depleted natural gas reservoirs by CO2 injection (van der Burgt et al., 1992; Blok et al., 1997; Oldenburg et al., 2001). The idea is to inject CO2 at some distance from producing wells and take advantage of the repressurization of the reservoir to produce additional CH4. The augmented methane production can be used to offset the cost of CO2 injection. We have termed this processFolk at the University of Kentucky have been looking at CO2 injection to push methane out of coal and black shale formations. A major part of the heating value of coal is in volatiles, but most of these are lost when the coal is pulverized for transport out of the mine. About 20 years ago, Eli tried to obtain a large block of coal to study this, only to find that in modern coal mines, the coal is broken into small pieces when cut deep in the mines or in the open pits.
CSEGR, or Carbon Sequestration with Enhanced Gas Recovery.
Comments
I researched oceanic carbon sequestration in the 1990's, but Greenpeace kept deep-sixing our field experiments (really!), first in Hawaii, then in Norway.
ReplyDeleteIncreasingly Eli's opinion on sequestration schemes is that you don't just throw carbon away, but you need to get something else out of the process.
ReplyDeleteWell, using CO2 for enhanced recovery is nothing new, so it's a pretty natural idea.
ReplyDeleteTrouble is that the "demonstrated integrity against gas leakage" was before the "existing infrastructure of wells" was put in place. Add any ground subsidence and you could have quite a leaky system. For example I have been involved in finding in-situ coal fires to depths of 450 m using surface gas measurements (radon). And its not remarkable - geochemists have long known that rapid vertical gas transport of up to 100's of metres per day can occur. try googling geogas + microbubble.
ReplyDeleteBruced
biochar is the way to go. in many soil regimes it provides fertility benefits as well as storing carbon.
ReplyDeleteHaven't they tried sequestration in Scotland and it leaked out.
ReplyDeleteI'm no science jockey, Eli, but the idea that you can pressure C02 in a hole in the ground and expect that it won't leak out is kinda silly.
And there's cost.
Eli, forget about this stuff as it's pie in he sky. The answer is fast breeder nuke. That's it. No stupid wind or silly solar panels. The final answer is nuke.
The point is that if you can get significant other value, especially energy, or displace other CO2 generating activity out of the CO2 from coal then leakage is not so concerning.
ReplyDeleteCanada (well Alberta really) is betting big time on CS. Canada's plan actually calls for electricity to be 90% non-CO2 emitting sources by 2020. That's really ambitious considering that most provinces still use a significant amount of coal-fired generation (BC and Quebec are major exceptions).
ReplyDeleteThere is some potential for use in depleted gas (and oil) wells in Alberta and even a demo project as I recall.
But you need to get the CO2 through a pipeline, I suppose, from the coal-fired plants that produce it to where it can be used.
Anyway, it's a race to the bottom, as Alberta's emissions from the oil/tar sands are expected to quintuple over the next decade or two.
Eek.
ReplyDeleteWhile it works well in some oil fields (where you can turn 30% oil recoveries into 50% oil recoveries), natural gas fields are a different story.
Presumably, in the big fields where they'd want to try this, you're probably already getting 90% recoveries or more. This means you're only going to recover a tiny fraction more of the gas in place when compared to oil. Further, gas sells at a much lower price than oil (on an energy equivalent basis) so there's very little financial incentive to do this.
If it's a gas field where recoveries are lower, it's normally a permeability issue. Thus, even if you inject CO2, those increased pressures will have a hard time spreading through the reservoir where they can meaningfully impact other wells.
Where sequestration has potential is in coalbed methane. Generally, more methane is adsorbed to the surfaces of the fractures in coal than there is floating around in the coal as free gas. However, CO2 has a higher affinity for being adsorbed to coal than methane, thus, by injecting CO2, it has the potential to replace adsorbed methane, freeing up the methane to flow out of the formation. The same idea has been proposed for methane hydrates, where CO2 has a higher affinity for being trapped in the crystal lattice than methane. Good luck getting the money to build a CO2 pipeline to the Arctic, however.
And indeed, Alberta is throwing all its hopes into one CCS basket. While there are no shortages of places to inject the CO2 and injecting acid gas has been done there for decades so the know how is already there, the capture process is another story.
CCS always seems to me in danger of becoming akin to perpetual motion - you burn hydrocarbons to get the energy to pump the CO2 from burning those hydrocarbons back into the ground to extract more hydrocarbons...
ReplyDeleteObviously, I don't know the numbers involved and they might be fantastic, but unless the energy cost of CCS is small (relative to the CO2 emissions of burning fossil fuels to get that energy) then it just seems like a waste of money.
Better to plant trees, bury biochar, and/or replace every coal plant with a nuke.
I suppose that - providing the carbon sequestered over carbon emitted ratio is > 1 - it might provide a slightly more palatable solution for entrenched industry interests - "we're not stopping anyone from burning coal, but you simply have to put all the CO2 straight back into the ground".
Pulverizing Mg2SiO4 and lifting it 5 km, so that wind can disperse it, costs about one-eighth of the electricity yield of the CO2 that it can then precipitate, if the C was in coal. Less than an eighth for fuels with more H per C.
ReplyDeleteNote: precipitate, not bury. MgCO3 can simply lie on the surface, and slowly dissolve and run into the sea.
(Taking into account the CO2 taken from air in order for one mole of CO3(2-) to dissolve as two moles of HCO3(-) reduces the energy fraction about twofold, from an eighth to a sixteenth.)
(How fire can be domesticated)
There are quite a few CO2-enhanced operation going on. In the absense of a price on CO2, it's clear why some companies are doing these things - squeezing out the last bit of profit AND gaining knowledge in even larger scale operations, for when the waves start spilling over the top of the sea wall. It'll be too late, but to say I'm disappointed would to deny what I know about people/ostriches and sand.
ReplyDeleteFollowing on from Miguelito, one case that you might like to look at is the K12B gas field in the North sea. Here the amount of CO2 in the natural gas, was too high (13%). Not that you can't use gas with this much CO2 in it, but there are regulations designed to keep the quality of gas being distributed more or less constant - yes, who would have guessed? Anyhow, at K12B, CO2 was being separated from the natural gas and vented to air. Some clever chap decided to try and improve the performance by injecting the CO2 back into the same reservoir. The field is running down, and the pressure is much lower than when production started - not much rocket science here. However, there are two points that are not so easy to learn about in this small project:
1. no enhancement of production was seen
2. CO2 concentration in the natural gas is increasing. (I heard 16%, but that's probably just hearsay or heresy.)
Go figure. Perpetual motion indeed. It's not all doom and gloom though. There's a lot to be said for enhanced-recovery techniques even if it's just to gain knowledge on how to do it. In Norway, at Sleipner, the CO2 from the natural gas is being injected at a much higher rate into a different formation. The reason for doing this: a Norwegian tax on the production of off-shore CO2.
There's been a bit of stink on the effect of the CO2 capture techniques used at these location recently, due to possible detrimental effects on marine life. Let me stress it's not the CO2 itself, but the current technology to separate it that could be the problem, i.e. in these two cases above the use of amines. There heresy is that the Norwegians are considerind banning some popular amines, but my Norwegian reading skills are poor. Maybe a rat out there could shed some light?
Apologies for high heresy content.
Bocco.
GRLCowan,
ReplyDeleteHow exactly are you going to get that magnesium sillicate to 5km height? Plane, train, automobile, particle accelerator?
The potential energy comes in at ~0.04 GJ/ton CO2 sequestered, which is actually a hundred times better than some current technologies at 3-4GJ/ton. (For your sake I'll make the assumption that this actually works). But it's not just pure potential energy that has to be overcome. As Kryton once said "Grind those balls, Sir!" That one eighth you mention means some pretty big particles - hope they'll still be reactive.
If we were to use a 5km high building with a lift. How much potential energy would you waste even putting up the building in the first place? Or did you have a 5km high magnesium-silicate mountain in mind?
And where are we going to inject? Somewhere where no-one lives and it's hot and humid. Erm, Venus? Nope, hot I give you, large driving force too with that atmosphere, but too dry, and definately above our 5km target. Ah the tropic, I'm sure the people there won't mind being in a contant mist of particles, coming from a material known to be found with asbestos. Remember, particles landing where it isn't hot and humid are going to do much. And as a bet, particles landing on an icesheet will only help the melt.
Oh here's a crazy idea, what about putting those particle in a hot, humid, high CO2 concentration of a power plant. Yes your right, a crazy idea... we're better off blowing caution to the wind.
</end sarcasm> It's my bad hair day.
Bocco.
"what about putting those particle in a hot, humid, high CO2 concentration of a power plant. Yes your right, a crazy idea"
ReplyDeleteIt is one of the ideas published by Dr. R.D. Schuiling. Perhaps you could find the reference, I don't have it handy. From my notes for May 29, 2008,
" C + O2 + (1/2) Mg2SiO4 ---> MgCO3 + (1/2) SiO2
Delta 'G' should be the sum of -33.055 kJ/mol for the olivine reaction and the free energy of formation of CO2 ... 1.0838 times the value when olivine is not involved."
That is to say, you get a little extra convertible energy by condensing the CO2, just as Rabett requires.
(How fire can be domesticated)
GRLCowan, that was my point, using olivine/serpetine/asbestos in a power plant exhaust where concentration of CO2 in can be between 3 and 15% ("best" gas to "worst" coal). That is going to be a lot more efficient than doing the reaction at 400 ppm CO2.
ReplyDeleteUnfortunately the gain from using the delta H (delta G tells you if it's going to work at all), which is also nicely negative, isn't that much. Realistically it'll produce only a small amount of medium to high pressure steam at somewhere between 200 (less useful) and 400°C (more useful). However, I've yet to be convinced that it won't be Red Dwarved by the amount of energy needed to "Grind those balls, Sir".
I haven't looked for a while, but Schuiling although undoubtedly an excellent geo-scientist has no peer reviewed papers about this subject I can easily find. Although I have seen several posters and popular science articles. The two people I have seen literature from on this subject (dust spreading) conclude that more research is needed, but it's definately not feasible in moderate northern climes. But these papers are more concerned with reaction rates than logistics.
It would be great if this worked, don't get me wrong. But all CO2 capture technologies cost energy, there's no free ride, even with these materials. Getting extra oil is just a temporary way of winning back investments and learning - waiting for the time of enforced CO2 sequestration. Of course if the world become oil constrained and nobody does sequestration, it still a win for the oil companies doing it.
You can easily work out how long it would take the biggest truck in the world, to cover 360 tonnes of pre-ground olivine into a 1 micron thick layer at 64 kmh using a 2.7 MW engine (assume it's running on CH4 for all the difference it will make). One suggestion was to use the Russian Steppe (shame that's probably too far north). Assume the truck is able to spread in a 100m wide track with no energy penalty. It'll put things in perspective.
Obviously, the idea of spreading from 5km high would be better, but I just can't imagine a feasible system to do that. Can you?
Bocco.
"Obviously, the idea of spreading from 5km high would be better, but I just can't imagine a feasible system to do that. Can you?"
ReplyDeleteJust can't? What were the fatal flaws in your three closest-to-feasible attempts?
"Just can't?"
ReplyDeleteNo free lunches from me. Why don't you try to calculate an estimate for the numbers involved.
Start with: How much primary energy does the monster truck use in the example above? How long does it take? How many monster trucks do you need to spread the dust equivalent of a small 1GW coal-fired power plant per year. What's the surface area of land your going to cover each year. What the cost of that land? Give an estimate of cost per tonne CO2 based on just trucks, free dust and land price. Assume the truck drivers are doing this out the goodness of their heart, or are actually made to do this by something more sinister. And show your working, LOL. This will earn you credits for Monster Truck Use 101.
Of course, there are other more interesting challenges to take at Bunny University. The top Bunny may or may not be aware that there's a whole lot of Bunny University Merchandise out there.
Unable to take up the challenge? No problem, someone has done it for you.
ReplyDeleteNuff said. Shame on me for having missed this.
Bocco.
"Let me stress it's not the CO2 itself, but the current technology to separate it that could be the problem, i.e. in these two cases above the use of amines. There heresy is that the Norwegians are considerind banning some popular amines, but my Norwegian reading skills are poor. Maybe a rat out there could shed some light?"
ReplyDeleteThe Norwegians are using some pretty complex chemical engineering there to fit a CO2 absorber onto a oil rig platform (like a membrane contactor). They may have had to compromise on some amine emissions because of that, or had to use one of the more volatile amines.
It would be a bummer if certain amines got banned for CO2 sequestration, as that means fewer degrees of freedom on how to design your absoprtion/desorption cycle to minimize the energy loss.
Very true, which is one of the attractions for grabbing the CO2 at the well head and the smokestack. In some cases you want to separate the CO2 anyhow, such as in natural gas before you put it into the distribution system.
ReplyDeleteAn interesting factoid that Eli picked up at the ACS conference is the cost of CO2 separated from natural gas is ~$25/ton, much lower than the cost of separation from the air (order of magnitude or more).