A couple of days ago Eli posted two MODTRAN calculations showing what would be seen at 70 km with CO2 for 375 ppm and 37500 ppm CO2 (Eli is a lazy bunny, he just added two zeros to 375)

and asked for an explanation of the donut hole when the CO2 mixing ratio is real high. The answer turns out to be both simple and a key to understanding the greenhouse effect. The clue was that temperature in this simulation decreased from 300 K at the surface to ~190 K at the 18 km tropopause and then increased again in the stratosphere up to where the ozone concentration was highest at ~30 km.
The answer is that at any frequency you are looking at emission from molecules (or aerosols) that are at the level where the emission can reach the detector without being absorbed again. If there is no absorption at a particular frequency you are looking at emission from the surface.
If the concentration is very high, this level moves up for any absorption band. If it moves up higher than the tropopause the temperature at which the CO2 emission that reaches you was emitted will be warmer than for lower CO2 concentrations. Take a look at the emission seen at 70 km if the concentration was 3.75 ppm

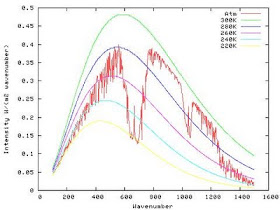
In this case the strong Q-branch (the sharp part pointing downwards in the middle of the CO2 bending absorption, reaches down to about the 220 K Planck function, indicating that the emission at that wavelength is so strongly absorbed, that one has to get to about 12 km where the temperature is ~220K before the emission would reach a detector at 70 km altitude. Other parts of the band absorb more weakly, and thus the emission that reaches the detector comes from lower down in the atmosphere.
If the CO2 mixing ratio is 37500 ppm then the absorption is so strong that to reach our detector at 70km, it has to be emitted from relatively high in the stratosphere, between 25 and 30 km where the temperature is above 240 K. In the wings of the band, where the absorption is weaker, the emitting level sits below or at the tropopause. Thus the donut hole. Even in the 375 ppm spectra, the Q branch emission comes from above the tropopause as can be seen by the sharp line pointing upwards at the position of the Q branch.
A
 nother way of looking at this is to look at the emission seen at 10 km/375 ppm where the temperature is ~240 K. As can be seen to the left, the center of the line extends down to ~240 K.
nother way of looking at this is to look at the emission seen at 10 km/375 ppm where the temperature is ~240 K. As can be seen to the left, the center of the line extends down to ~240 K.There are a bunch of caveats here, the most important of which is that the MODTRAN temperature profile is not adjusted to take feedbacks into account. As raypierre says, if we really stepped the CO2 up to 37500 ppm, all hell would break loose and the temperature profile would in the stratosphere would cool "hiding the blip", a good trick. Bunnies should also note that the CO2 band widens as the concentration increases. This accounts for a great deal of the greenhouse effect.
The idea is very useful for understanding the greenhouse effect.
Let's do more exercises like this. It's rather more fun than arguing about nothing.
ReplyDeleteThis was nice. By 'km' before the 3rd image you probably mean 'ppm', but I don't see it said anywhere, so an A-. I got an E+ because my answer was "Probably warmer CO2 in the upper layers is more prone to absorb radiation".
ReplyDeleteI fondly remember the Christmas (or was it Channukah) that Eli gave me the MODTRAN pony - or at least a link to it. Playing with it and understanding what one sees teaches a lot about the GH.
ReplyDeleteMarkeyMouse says: But the Wood experiment shows that trapping IR, (which is the mechanism AGW theory say by which CO2 does warming) has in reality no material effect on atmospheric temperature, therefor more or less CO2 absorbing IR and releasing at other wavelngths doesn't affect temperature. End of AGW theory. Sorry Bunny. Back to the Woodshed for you.
ReplyDeletehttp://www.giurfa.com/gh_experiments.pdf
MM,
ReplyDeleteAll the Wood experiment shows is that glass greenhouses don't trap ir. They restrict convection. This is common knowledge.
Try this at home:
http://www.espere.net/Unitedkingdom/water/uk_watexpgreenhouse.htm
The biggest caveat to accepting the CO2 theory is the 0.2w/m^2 GW effect that shows on your graph. This quarter watt of (eventually heated warmer air) has to heat the Earth, heat the miles deep oceans and melt the Arctic whilst leaving the Antarctic alone.
ReplyDeleteluminous beauty,
ReplyDeleteThe Wood experiment is clearly flawed, as was pointed out in a not much later issue by none other than Charles Greeley Abbot. A glass greenhouse does trap IR, but unless it's double or triple glazed, the insulation is so bad that the effect would be very small. Consider low E glass as another counter example.
Wood's experiment is so poorly documented, it's impossible to duplicate. The most likely reason he found the results he did is that the boxes were not similarly insulated. That could have been checked and reported at the time, but wasn't. Now some people would say that Wood was such a great experimentalist that he must have done that. To which I reply, if it wasn't in the paper, it didn't happen.
Looking for a bit of clarity.
ReplyDeleteHow can the emission of 15um become stronger than the 240K temp set by top of the troposphere?
There's a supply of photons coming from the surface being absorbed and then emitted by co2 at a rate dependant upon gas temp. Since the 15um must first clear the troposphere I don't understand how it can then be emitted at a higher rate. I understand the stratosphere being warmer but the supply to it is coming from a cold layer.
One thought I had is that there is maybe a broader level absorption (ex 14.5-15.5) but an emission much closer to 15 (14.9-15.1) which would explain how the intensity could increase.
Guidance and patience appreciated.
If you are observing the IR emission from space you see the emission from the level at which the absorption of CO2 becomes low enough that an emitted photon can reach space without being reabsorbed. At current concentrations this is only at the center of the 15 u band, the sharp spike in the first spectrum. At other wavelengths the emission from the troposphere overwhelms that from the stratosphere.
ReplyDeleteThe net effect of CO2 emission in the stratosphere is to cool the stratosphere, a good explanation can be found here:
https://web.archive.org/web/20150203172753/http://www.atmosphere.mpg.de/enid/20c.html
Hope that works for you
Thanks,
ReplyDeleteI think that does help a bit. I was having a conservation of energy issue. The stratosphere cooling is the consequence and source for the missing energy.